Medical Terminology Daily (MTD) is a blog sponsored by Clinical Anatomy Associates, Inc. as a service to the medical community. We post anatomical, medical or surgical terms, their meaning and usage, as well as biographical notes on anatomists, surgeons, and researchers through the ages. Be warned that some of the images used depict human anatomical specimens.
You are welcome to submit questions and suggestions using our "Contact Us" form. The information on this blog follows the terms on our "Privacy and Security Statement" and cannot be construed as medical guidance or instructions for treatment.
We have 1256 guests and no members online
Jean George Bachmann
(1877 – 1959)
French physician–physiologist whose experimental work in the early twentieth century provided the first clear functional description of a preferential interatrial conduction pathway. This structure, eponymically named “Bachmann’s bundle”, plays a central role in normal atrial activation and in the pathophysiology of interatrial block and atrial arrhythmias.
As a young man, Bachmann served as a merchant sailor, crossing the Atlantic multiple times. He emigrated to the United States in 1902 and earned his medical degree at the top of his class from Jefferson Medical College in Philadelphia in 1907. He stayed at this Medical College as a demonstrator and physiologist. In 1910, he joined Emory University in Atlanta. Between 1917 -1918 he served as a medical officer in the US Army. He retired from Emory in 1947 and continued his private medical practice until his death in 1959.
On the personal side, Bachmann was a man of many talents: a polyglot, he was fluent in German, French, Spanish and English. He was a chef in his own right and occasionally worked as a chef in international hotels. In fact, he paid his tuition at Jefferson Medical College, working both as a chef and as a language tutor.
The intrinsic cardiac conduction system was a major focus of cardiovascular research in the late nineteenth and early twentieth centuries. The atrioventricular (AV) node was discovered and described by Sunao Tawara and Karl Albert Aschoff in 1906, and the sinoatrial node by Arthur Keith and Martin Flack in 1907.
While the connections that distribute the electrical impulse from the AV node to the ventricles were known through the works of Wilhelm His Jr, in 1893 and Jan Evangelista Purkinje in 1839, the mechanism by which electrical impulses spread between the atria remained uncertain.
In 1916 Bachmann published a paper titled “The Inter-Auricular Time Interval” in the American Journal of Physiology. Bachmann measured activation times between the right and left atria and demonstrated that interruption of a distinct anterior interatrial muscular band resulted in delayed left atrial activation. He concluded that this band constituted the principal route for rapid interatrial conduction.
Subsequent anatomical and electrophysiological studies confirmed the importance of the structure described by Bachmann, which came to bear his name. Bachmann’s bundle is now recognized as a key determinant of atrial activation patterns, and its dysfunction is associated with interatrial block, atrial fibrillation, and abnormal P-wave morphology. His work remains foundational in both basic cardiac anatomy and clinical electrophysiology.
Sources and references
1. Bachmann G. “The inter-auricular time interval”. Am J Physiol. 1916;41:309–320.
2. Hurst JW. “Profiles in Cardiology: Jean George Bachmann (1877–1959)”. Clin Cardiol. 1987;10:185–187.
3. Lemery R, Guiraudon G, Veinot JP. “Anatomic description of Bachmann’s bundle and its relation to the atrial septum”. Am J Cardiol. 2003;91:148–152.
4. "Remembering the canonical discoverers of the core components of the mammalian cardiac conduction system: Keith and Flack, Aschoff and Tawara, His, and Purkinje" Icilio Cavero and Henry Holzgrefe Advances in Physiology Education 2022 46:4, 549-579.
5. Knol WG, de Vos CB, Crijns HJGM, et al. “The Bachmann bundle and interatrial conduction” Heart Rhythm. 2019;16:127–133.
6. “Iatrogenic biatrial flutter. The role of the Bachmann’s bundle” Constán E.; García F., Linde, A.. Complejo Hospitalario de Jaén, Jaén. Spain
7. Keith A, Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J Anat Physiol 41: 172–189, 1907.
"Clinical Anatomy Associates, Inc., and the contributors of "Medical Terminology Daily" wish to thank all individuals who donate their bodies and tissues for the advancement of education and research”.
Click here for more information
- Details
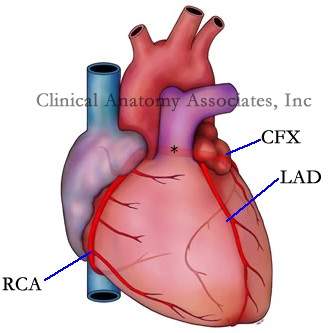
The term [coronary] comes from the Latin root [corona] meaning "crown", therefore [coronary] is used to denote a structure that surrounds another as a crown or a garland. In the heart, the coronary arteries and their branches form a crown that surrounds the heart at the level of the atrioventricular sulcus. There are two coronary arteries, which arises from, the root of the aorta; the right coronary artery (RCA), and the left coronary artery (*). The two main branches that arise from the left coronary artery are the circumflex artery (CFX) and the left anterior descending artery (LAD).
There can be interesting anatomical variations in the coronary arteries of the heart.
Although not in use anymore, the gastric arteries used to be called the "gastric coronaries" as the right and left gastric arteries and the right and left gastroepiploic arteries form a garland of arteries that surround the stomach. The term still does apply to the left gastric veins.
- Details
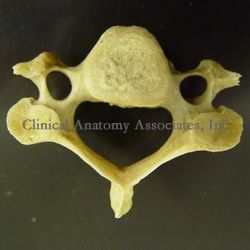
From the Latin [vertere] meaning "to turn", the term refers to one of the bones that forms the spinal column or raquis. This word was first used by Celsus both to denote the intervertebral joint and the bone itself. The plural form of the term [vertebra] is [vertebrae].
All vertebrae are different, although they have some similarities which allows us to group them by region: cervical, thoracic, lumbar, sacral, and coccygeal. The image shows us a cervical vertebra, characterized by a slender, small vertebral body and two lateral openings, the transverse foramina. If you hover your cursor over the image, a thoracic vertebra will appear. Thoracic vertebrae are characterized by a heart-shaped body, the presence of articular surfaces for the ribs, etc. With few exceptions, all vertebrae have a basivertebral foramen.
Photography by D.M.Klein
- Details
This article is part of the series "A Moment in History" where we honor those who have contributed to the growth of medical knowledge in the areas of anatomy, medicine, surgery, and medical research.
Sir Arthur Keith (1866–1955) was a Scottish physician, anatomist and anthropologist. He studied medicine at the University of Aberdeen, earning his Bachelor of Medicine in 1888. He traveled to Siam and worked for three years as a medical officed in a rubber plantation and mine. Upon his return to London, he continued his medical studies at University College. He became a fellow of the Royal College of Surgeons of England in 1894.
Keith held various academic positions, including demonstrator of anatomy at the London Hospital, where his initial anatomical research expanded to include pathological specimens associated with clinical cardiology. His collaboration with Sir James Mackenzie (1853 – 1925), a cardiologist studying cardiac arrhythmias using polygraph tracings (a device used to study cardiac arrythmias before the invention of the electrocardiograph by William Einthoven (1860 – 1927), piqued Keith’s interest in the anatomical basis of heart rhythm disorders.
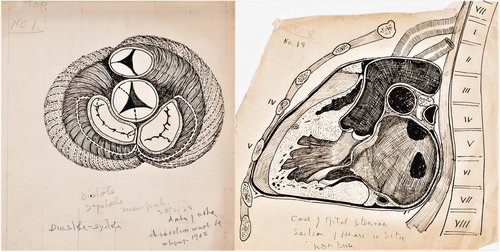
He was also an accomplished artist, and his anatomical illustrations and dissections helped clarify the structure and pathological variations of the atrioventricular conduction system. Through extensive dissection of post-mortem hearts sent by Mackenzie, Keith contributed to understanding the relationship between structural features of the conduction system and clinical manifestations of arrhythmias.
The discovery of the sinoatrial (SA) node is among Keith’s most enduring scientific legacies. Inspired by the 1906 work of Japanese anatomist Sunao Tawara, who described the atrioventricular node and conduction pathways in the mammalian heart, Keith and Martin W. Flack (a medical student at the time) extended this work in a search for the anatomical site responsible for initiating the heartbeat.
In 1906, while studying the heart of a mole, Flack identified a distinct structure at the junction of the superior vena cava and right atrium. Recognizing its histological resemblance to known conduction tissue, Keith and Flack named this structure the sino-auricular node. They hypothesized that this node was the dominant center initiating cardiac rhythm. Their work was published in 1907 in the Journal of Anatomy and Physiology. This publication completed the anatomical outline of the conduction system of the heart as we know it today.
Keith was appointed Conservator and Hunterian Professor at the Royal College of Surgeons in 1908 and transitioned his studies toward evolutionary anatomy and anthropology. He became an influential figure in paleoanthropology, published several books on human evolution, and served as President of the Royal Anthropological Institute and Rector of Aberdeen University. Knighted in 1921 and elected Fellow of the Royal Society in 1913, Keith remained active in research and writing until his death in 1955.
Sources:
1. Mohr PD. Illustrations of the heart by Arthur Keith: His work with James Mackenzie on the pathophysiology of the heart 1903–1908. J Med Biogr. 2021;30(3):193–201
2. Silverman ME, Hollman A. Discovery of the sinus node by Keith and Flack: on the centennial of their 1907 publication. Heart. 2007;93(10):1184–1187.
3. Keith A, Flack M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J Anat Physiol. 1907 Apr;41(Pt 3):172-89. PMID: 17232727; PMCID: PMC1289112.
4. Keith A, Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J Anat Physiol 1907;41: 172–189.
Images published in this article under SAGES permission on Creative Commons Attribution 4.0 LicenseOther images Wellcome Collection Gallerypermission on Creative Commons Attribution 4.0 License
- Details
- Hits: 5017
This article is coauthored by Randall K. Wolf, MD and Efrain A. Miranda, PhD
Can there exist a unified theory and mechanism for arrythmias and atrial fibrillation (AFib) that can explain the results of various catheter-based and surgical-based treatments? Based on anatomical and physiological study of the heart and it’s nervous system, we believe the answer is yes.
While we have learned not to expect everyone to be persuaded by the argument we will be presenting, we suggest, nevertheless, this argument does deserve careful and dispassionate consideration for it provides a unified model which explains results in the treatment of AFib, and should not be ignored. The epiphany that shed light on a possible unifying mechanism came during multiple cases of left atrial electrical testing during the conduct of minimally invasive surgical pulmonary vein isolation (PVI) in patients who had multiple previous catheter-based PVI procedures. These cases will be published separately.
Over the years we have evolved our view of the structures and processes that control the beating rhythmic activity of the heart. Additional and complementary information can be found following the links in the article.
First, we need to define two terms in reference to the heart: “Intrinsic” and “Extrinsic”. An intrinsic structure is found within the boundaries of the heart, that is, within the parietal pericardium. Extrinsic means that the structure about is found outside the parietal pericardium.
The most well-known intrinsic component is what we know as “the conduction system of the heart”. The classic description of the conduction system of the heart emphasizes this cardiomyocyte-based component and refers to a group of specialized cardiac muscle structures that serve as pacemakers and distributors of the electrical stimuli that make the heart beat coordinately. It is important to stress the fact that this primary "conduction system of the heart" is not formed by nerves but rather by specialized cardiac muscle cells. Probably the reason so much emphasis is placed on the conduction system of the heart is the use of the electrocardiogram as a clinical diagnostic tool since William Einthoven (1860 - 1927) introduced the EKG in 1901.
The second (quite complex) component of this system has been forced to take a secondary place, and in many cases ignored. We refer to the modulating activity on the heart by the autonomic nervous system (ANS), with its two subsystems, sympathetic and parasympathetic.
Unlike the conduction system of heart, which is purely intrinsic, the cardiac autonomic system is both extrinsic and intrinsic. The classic architecture described is a two-neuron system, where an extrinsic preganglionic neuron located in the central nervous system (CNS) connects with an extrinsic postganglionic neuron found in the sympathetic chain and ganglia located in the superficial and deep cardiac plexuses close to the aorta and pulmonary trunk.
The cardiac ANS has an intrinsic component formed by ganglionated plexi (GPs) and nerves found within the walls of the myocardium and in epicardial areas of the heart which contain fat. The main location of these GPs is close to and around the great vessels. These ganglionated plexi are the basis for the complex rhythmicity responses of the hear. In fact, several researchers call this intracardiac plexuses the "Little Brain of the Heart". Failure of the ganglionated plexi are the basis of many cardiac arrythmias, including atrial fibrillation.
The inclusion of the cardiac ganglionated plexi into this picture has led us to propose a different ANS organization for those organs that have rhythmicity, be it a beat (heart) or peristalsis (digestive system, ureters, urethra, etc.). For a more detailed explanation follow this link to another article in this website.
These cardiac intrinsic ANS component are responsible for the complex reflexes that increase or decrease both the heartbeat and the force of contraction of the heart muscle in response to variations in volumetric pressure in the atria and chemical variations in the blood caused by alcohol, caffeine, drugs, dehydration, etc. A more detailed explanation can be found in the following Houston Methodist DeBakey CV Live video. Click on the video to start it or you can go directly to YouTube by clicking here. The main content start at 2 minutes:
The cardiac ANS has important communication with the brain centers responsible for mood and emotions. It is important to note that emotional response is linked to visceral activity (glands and viscera), and this includes the heart.
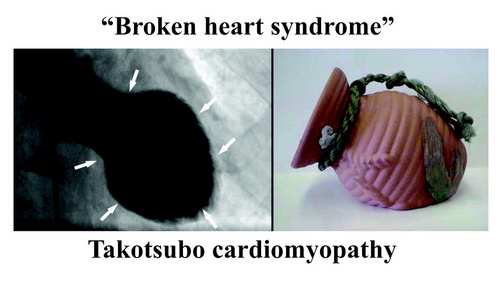
Also, the ANS/GPs complex is not separated from the higher functions of the forebrain. Cechetto (2005) explains how the forebrain (conscious) activity influences the spinal cord and the ANS by pathways that include the limbic system, insula, amygdala, and lateral hypothalamus. These pathways and communications can certainly explain arrythmias caused by stress and anxiety and pathologies such as the “broken heart syndrome” (Takotsubo cardiomyopathy).
Note: "Broken heart syndrome" or "stress cardiomyopathy" is also known as "Takotsubo cardiomyopathy". This is because the shape of the heart in this condition changes and resemble a Japanese octopus (tako) trap (tsubo). For more information on this condition, click here.
Sources and references
1. Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (2005) 209: 425–438
2. D. F. Cechetto, "Forebrain Control of Healthy and Diseased Hearts," Chapter in "Basic and Clinical Neurocardiology", J. Armour and J. L. Ardell, Eds., Oxford University Press, 2004.
3. Kandel ER, Koester JD, Mack SH, Siegelbaum SA. Principles of Neural Science. 6th ed. New York, NY: McGraw Hill; 2021.
4. Standring S, ed. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 42nd ed. London, UK: Elsevier; 2021.
5. Haines DE, Mihailoff GA. Fundamental Neuroscience for Basic and Clinical Applications. 5th ed. Philadelphia, PA: Elsevier; 2018.
6. Guyton AC, Hall JE. Textbook of Medical Physiology. 14th ed. Philadelphia, PA: Elsevier; 2021.
7. Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience. 6th ed. New York, NY: Oxford University Press; 2018.
8. Felten DL, Maida MS. Netter’s Atlas of Neuroscience. 4th ed. Philadelphia, PA: Elsevier; 2021.
9. Wolf, RK; Miranda, EA. Minimally Invasive Surgical Treatment of Atrial Fibrillation: A New Look at an Old Problem. 2024 Operative Techniques in Thoracic and Cardiovascular Surgery.
10. Takotsubo Cardiomyopathy Harvard.edu