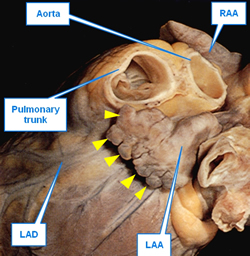
UPDATED: The left atrial appendage (LAA) is an embryological remnant of the development of the heart. It represents the primitive left atrium (LA) which is then “pushed to the side” by the development of the final (adult) stage of the LA. While the LAA is thin, tubular, tortuous, and presents with convoluted muscular walls, the adult LA has smooth walls and is considered to be a dilation of the terminal portion of the veins that enter the LA, hence the name “sinus venarum”, another term for the atria. The LAA is also known as the "left atrial appendix", or the "left auricle".
The anatomy of the LAA is presented in the video included at the end of the article, but there are some details that are important to discuss in the involvement of the LAA in the creation of thrombi and emboli in the presence of atrial fibrillation (AFib).
LAA shape and size
The LAA has important anatomical variations, with different shapes that anatomists and physicians have tried to consolidate in groups such as: chicken wing, cactus, windsock, cauliflower, etc. The fact is that recording the shape of the LAA is subjective. as the evaluation depends completely on the observer.
Researchers have tried to determine what shape can lead to a higher potential for stroke-producing emboli when AFib is present. A recent study by Dudzińska-Szczerba (2021) and an editorial by Yong Shin (2021) states that the shape itself is not a good predictor, but the distance between the LAA ostium and the first bend of the LAA is indeed a good predictor. The longer the distance there is increased potential for thrombus and emboli formation.
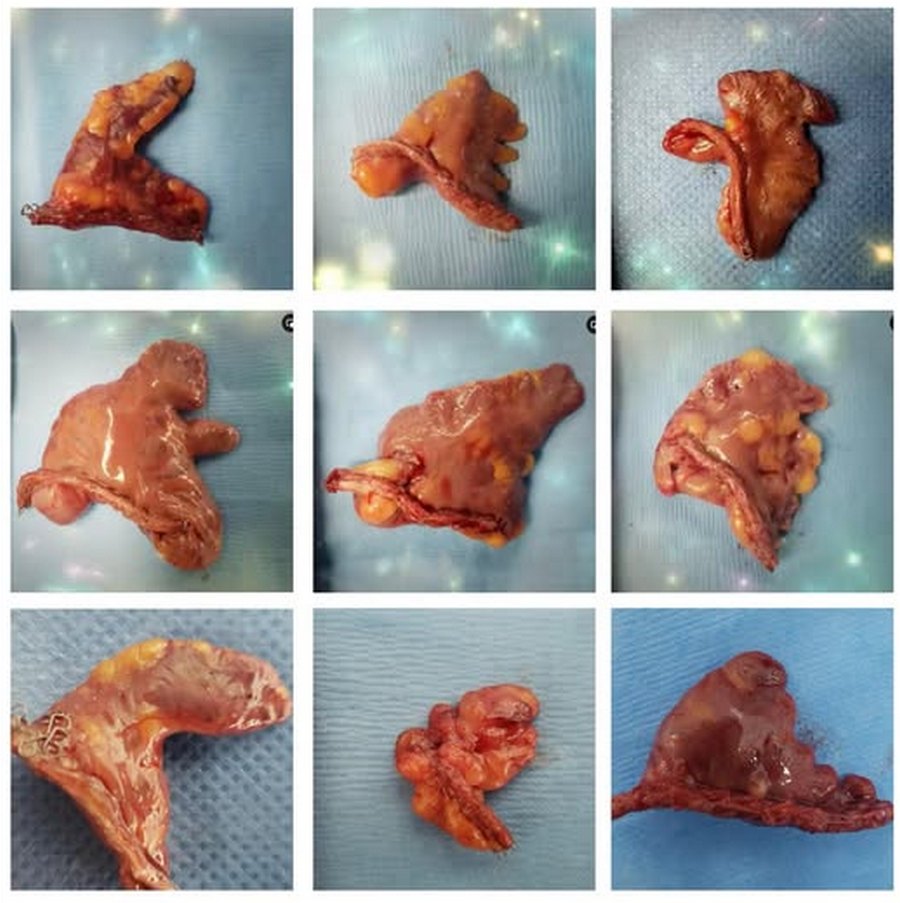
Left atrial appendage shapes. Courtesy of Sandra Fezzat Schrameyer
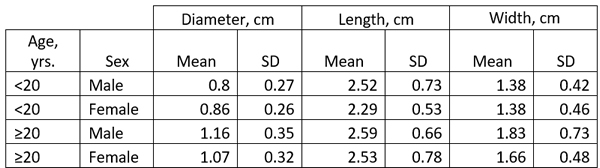
The size of the LAA has been studied in detail and it ranges ranging from 0.3 cm to 2.0 cm in males, and 0.3 to 1.8 cm in females. (Venoit, 1997), as shown in this table.
The LAA ostium
The LAA ostium is the communication between the LA and the LAA, it is generally oval in shape and its size is variable. The ostium is in some cases slit-like, or an elliptical-shaped variant, “smiley”, and even small circular (DeSimone, 2015; Cabrera, 2014). A study by Wang (2010) classified the LAA ostium into five types: oval (68.9%), foot-like (10%), triangular (7.7%), water drop-like (7.7%), and round (5.7%). It is interesting that devices that are used to occlude the LAA ostium are round and that is only 6% of the population reported in the Wang (2010) study. In a study by Su (2006) it was found that 100% of the specimens studied the LAA ostium had an oval shape with the mean diameter of the opening of 17.4 mm with a range between 10-24.1 mm).
In reference to LAA ostium occluders Su (2006) states that "These percutaneous devices / systems,however, have a round shape to fill or cover the LAA ostium. A previous study and our study show that the shape of the LAA ostium is consistently elliptical rather than round. This suggests that to seal the LAA orifice adequately without oversizing, devices may need to be elliptical for a snug fit. A round implant over an oval-shaped orifice may leave crevices on either side of the implant, leading to incomplete sealing of the orifice."
Lobes
The LAA can also present with different dilations called “lobes” these can range from zero to three or four.
Muscular Wall Structure
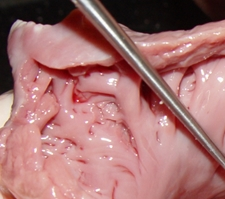
The LAA has internal ridges that form a muscular meshwork. The term used to describe these is “trabeculated”. It makes sense that in the case of atrial fibrillation, the slow to non-existent flow of blood within the deep recesses of the trabeculated muscular wall of the LAA will cause blood to pool and coagulate, forming thrombi. The presence of these LAA trabeculations have been found to be associated with stroke risk by Dudzińska-Szczerba (2021). The accompanying image shows the trabeculations in a cow's LAA. They are not as deep or as convoluted as those found in a human heart.
Crenellations
This is a rarely used term. It is a pattern along the top of a fortified wall, as in a castle, forming multiple, regular, rectangular spaces. These crenellations are found in the edge of the LAA compounding the irregularity of the internal wall and increasing the chance for thrombus formation and stroke-inducing emboli. Crenellations are shown by yellow triangles in the first image in this article.
Function of the LAA
As stated, the LAA is an embryological remnant, but it does have a function in the adult. It generates a peptide involved in the control of salt in the circulatory system. This is the atrial natriuretic peptide (ANP), a hormone that is secreted by both the right and left atria and their appendages in response to circulatory volume and pressure changes. ANP helps the elimination of excess sodium through the kidneys (natriuresis), control of urine elimination (diuresis), and antifibrotic and antihypertrophic effects within the heart (Sandeur, 2023)
While removing both the right and left atrial appendages could cause ANP deficiency, surgical removal or exclusion of only the LAA does not cause an ANP problem (8).
Involvement of the LAA in AFib
The LAA is an electrically active structure. The cardiomyocytes that form its walls have automatic activity and it has been described as an area that can trigger AFib. The accompanying video shows an LAA that has been separated from the heart (in this case using a surgical stapler) and it can be seen how the LAA continues fibrillating on its own. Video courtesy of Dr. Randall K. Wolf
This is the why LAA exclusion is a must in the case of AFib and potentially in any cardiovascular procedure where the pericardial sac is opened (this is a subject for discussion).
The problem is that devices that only occlude the LAA ostium do not electrically isolate the LAA wall from the LA wall, leaving this potential AFib-producing connection intact. Just occluding the LAA ostium is not a solution for atrial fibrillation, it just reduces the risk of stroke.
Personal note: In May 5th, 2020 Dr. Randall K. Wolf invited me to a live webcast where we reviewed the anatomy of the left atrial appendage, the problems the LAA can cause in atrial fibrillation leading to stroke, and the reasons for its exclusion in AFib surgery. This video is next. You can watch other videos on the topic here. Dr. Miranda.
Sources:
1. “Anatomy of the Normal Left Atrial Appendage: A Quantitative Study of Age-Related Changes in 500 Autopsy Hearts: Implications for Echocardiographic Examination” Veinot, JP; et al. 1997 Circulation; 96:3112–3115
2. “A Review of the Relevant Embryology, Pathohistology, and Anatomy of the Left Atrial Appendage for the Invasive Cardiac Electrophysiologist” De Simone, CV, et al. J AFib 2015; 8:2 81-87
3. “Left atrial appendage: anatomy and imaging landmarks pertinent to percutaneous transcatheter occlusion” Cabrera,JA; Saremi, F; Sanchez-Quintana, D. 2014 Heart 2014 100:1636-1650
4. Left Atrial Appendage Studied by Computed Tomography to Help Planning for Appendage Closure Device Placement” Wang Y. et al. J Cardiovasc Electrophyisiol 2010 21:9 973-982
5. IIs the Left Atrial Appendage (LAA) anatomical shape really meaningless measure for stroke risk assessment? Shin, SS; Park, JW. Int J Cardiol 2021 May 1:330:80-81. doi: 10.1016/j.ijcard.2021.02.047.6. “Assessment of the left atrial appendage morphology in patients after ischemic stroke” Dudzińska-Szczerba, K. et al. Int J Cardiol 2021 330:65-72
7. “Atrial Natriuretic Peptide” Sandeur, CC; Jialal, I. Stat Pearls 2023. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK562257/
8. Personal communication, Dr. R. Wolf 2023
9. "Slide Atlas of Human Anatomy" Gosling, J.A.; Whitmore, I; Harris, P.F.; Humpherson, J.R., Et al; ISBN: 0723426570 Hong Kong: Times Mirror, 1996
10. "Atrial and brain natriuretic peptides: Hormones secreted from the heart" Nakagawa Y, Nishikimi T, Kuwahara K. Peptides. 2019 Jan;111:18-25.
11. "Occluding the left atrial appendage: anatomical considerations" Su, P; McCarthy, KP; Ho, SY. 2008 Heart 94:1166–1170
Thanks to Sandra Fezzat Schrameyer for the image of the different shapes of the LAA.